
In: Recent Advances in Small Animal Reproduction, P. W. Concannon, G. England and J. Verstegen (Eds.) Publisher: International Veterinary Information Service (www.ivis.org), Ithaca, New York, USA.
Intra-Uterine Insemination in the Dog Using the Scandinavian Trans-Cervical Catheter and a Comparison with other Methods ( 2-Feb-2001 )
C. Linde-Forsberg
Faculty of Veterinary Medicine, Department of Obstetrics and Gynaecology, Swedish University of Agricultural Sciences, Uppsala, Sweden
Introduction
The interest for canine semen preservation and artificial insemination (AI) is steadily increasing world-wide, yet comparatively few studies are published each year within this field, and it is particularly difficult to find data from artificial inseminations using fresh semen, or semen preserved according to various methods for chilling, or freezing and thawing, and also comparing results when different techniques to perform the inseminations are used. One reason for the scarcity of data is that it is expensive to do experimental studies in dogs, and few research centers can keep large enough dog colonies for this kind of studies. Another reason is that most of the large companies processing and distributing canine semen for dog breeders are working on a purely commercial basis, and although some of them appear to keep records of their results neither these results, nor the composition of the extenders or the methods for preservation of the semen are disclosed. Any knowledge thus gained will, therefore, remain with the different companies, and will not be of benefit to the scientific world, and thus neither to the dog breeders in general. The only studies on canine artificial insemination reporting on fertility results from a large number of inseminations are those by Seager et al., [1] on 156 frozen-thawed semen AIs using vaginal deposition, Linde-Forsberg & Forsberg [2,3] on 470 and 527 AIs respectively, using both fresh, chilled extended and frozen-thawed semen and vaginal as well as intra-uterine AI, Linde-Forsberg et al., [4] on 327 frozen-thawed semen AIs, comparing vaginal and intra-uterine AI, Linde-Forsberg [5] reporting on 2041 AIs with fresh and chilled extended, and frozen-thawed semen and using both vaginal and intra-uterine AI, and Thomassen et al., [6] on 312 frozen-thawed mainly intra-uterine AIs. A number of factors are of importance in determining the success rate of artificial insemination in dogs, such as when and how many times during the estrous cycle of the bitch the AI is performed, semen quality and handling, and the insemination technique. More information about those facets can be found in the cited references. This paper will only deal with the insemination techniques and the effects of the site of semen deposition. The recent clinical studies [4,5] have highlighted the importance of intra-uterine as opposed to intravaginal artificial insemination in the dog and are the first to demonstrate significantly better results when semen was deposited in the uterus rather than in the vagina. In the study by Linde-Forsberg et al., [4] the whelping rate using intra-uterine deposition of frozenthawed semen was 84.4 % compared to 58.9 % using vaginal deposition, and mean litter size was 5.4 + 3.0 compared to 4.0
+2.7 pups/litter (P<0.001). Fertility data from 2041 artificial inseminations is dogs, performed in Swedish bitches between 1990 and 1998 and reported to the Kennel Club within 2 weeks, (i.e. before a pregnancy test could have been made to avoid bias to the results), showed that significantly higher whelping rates and litter sizes were obtained not only with frozen-thawed semen but also with fresh, as well as chilled, extended semen when the semen had been deposited in the uterus rather than in the vagina [5] (Table 1).

The results using fresh semen, shown in Table 1, are slightly too high because 11 % of those bitches were, for various reasons, not only artificially inseminated but also mated. The most frequent reason for this was that the breeders tried to mate the bitches too early during estrus and when the dogs would not mate, their owners requested an AI. The whelping rate in the also-mated group was as high as 84.5%, with a mean litter size of 6.0 + 2.8 pups, compared to a whelping rate of 48.9% and a mean litter size of 5.8 + 2.8 pups for those bitches that were only artificially inseminated. It appears, therefore, that a number of the AIs that are performed with fresh semen actually are done at a non-optimal time during estrus, usually because of the breeder‘s inexperience, but the comparatively poor result is likely also due to that AI is requested because of various problems in the dogs. The Swedish data [5], however, clearly demonstrate that in the dog, intra-uterine AI significantly improves the whelping rate and litter size. The whelping rate by intra-uterine AI increased with from 36% for fresh semen to 50% for frozen-thawed semen, compared to vaginal AI. And, with intra-uterine AI, the mean litter size increased by 0.3 pups per litter for frozenthawed semen and by 0.6 and 0.7 per litter for fresh, and chilled extended semen. It is also interesting in this context to note that the dog is considered to be a species with intra-uterine deposition of semen at natural mating, because by the time the copulatory tie is over, the spermatozoa have reached the oviducts [7]. The sperm rich fraction is ejaculated at intromission and during the early stage of the copulatory tie, and is followed by a large volume of prostatic fluid which immediately flushes the spermatozoa from the narrow cranial vagina through the cervix into the uterine body and horns. The vagina appears to offer an unfavorable environment for canine spermatozoa, since a large proportion loose their tails within minutes after deposition in the cranial vagina [8]. It has also been shown that around 10 times as many spermatozoa are required to obtain similar results by vaginal AI as by intra-uterine AI with fresh semen [9], as well as with frozen-thawed semen [4]. There are, thus, many factors which contribute to the poorer results obtained by intravaginal semen deposition compared to intra-uterine deposition.
Methods to Perform Intra-Uterine AI in the Dog.
Intra-uterine AI in the dog can be done transcervically either by way of the Scandinavian (or Norwegian) catheter, or by using a rigid fiberoptic vaginal endoscope to visualize the cervix and a dog urinary catheter to transverse it. Intra-uterine AI can also be accomplished by invasive methods such as laparoscopy, or full abdominal surgery. In some countries the latter methods may be illegal or not considered ethically acceptable.
Palpation of the Cervix
It is absolutely essential for the person who wishes to perform canine AI to learn how to locate the cervix by abdominal palpation in order to be able to deposit the semen in the correct place and to avoid injuring the bitch. The bitch should have an empty stomach and bladder to facilitate the procedure. For training purposes it is recommended to use the single-use plastic canine vaginal AI-catheters (Minitüb GmbH, Tiefenbach, Germany) (Fig. 1).
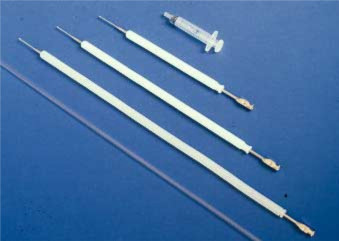
Figure 1. The three sizes of the Scandinavian artificial insemination catheter for dogs, and the plastic single-use vaginal artificial insemination catheter.
Because the uretheral opening of the bitch is located at the pelvic brim, it is surprisingly easy for the AI catheter, or a thin rigid endoscope, to be unintentionally introduced into the urinary bladder. Apart from the hazards of perforating the bladder with the catheter, it is obvious that no pregnancy would follow after an AI. Thus, the correct position of the catheter should always be checked by palpation before depositing a semen dose. If the catheter is in the urinary bladder, the cranial part of the vagina and the cervix can be palpated above the catheter. The walls of the urinary bladder usually are thinner than those of the vagina, and the tip of the catheter stands out more distinctly than if it were in the vagina. To palpate the cervix, an AI catheter is introduced into the vagina of the bitch. The introduction of the catheter is facilitated if the vulva is elevated until it is just below the anus (like when the bitch stands for the male dog). When the tip of the catheter is introduced as far as to immediately cranial to the pelvic brim, it should be palpated. Cranially the vagina in most bitches slopes slightly downward. In some breeds, however, especially the sight hounds with a very arched loin, the vagina has a more dorsal direction. The cranial end of the catheter should now be lowered closer to the abdominal wall to become more accessible to palpation. When the catheter tip can be palpated and its correct position in the vagina thus checked, it is carefully introduced further, under continued palpatory control, until it reaches the paracervical area. This is the narrow, cranial portion of the vagina created by the dorsal, median post-cervical fold and can be palpated as a 1 to 2 cm long, firm structure. It ends at the cervix, which in a bitch in estrus is a 0.5 to 1.5 cm hard, rounded-to-ovoid freely movable structure. It is usually not possible to pass the outer protecting sheath of the Scandinavian catheter, which has a diameter of 10 mm, into the paracervical area. Also the thinner plastic AI catheter, which has a diameter of 5 mm, may be too wide to introduce into the paracervical area in some bitches, especially those of the smaller breeds, or those that have not given birth to a litter of pups. Once the cervix has been identified the corpus uteri and the uterine horns can be palpated in front of this structure. Lower the tip of the catheter and then close the tip of the thumb against that of the index finger above the catheter, then lift the cranial end of the catheter in such a way that the cervix and the uterine horns are pulled upward between the fingers. Their size and consistency then become evident. (This method of palpating the uterus is also very useful for early pregnancy detection and to examine bitches with suspected pyometra).
Intra-Uterine Insemination using the Scandinavian Catheter
The Scandinavian catheter consists of a 1 – 2 mm wide steel catheter with a 0.75 mm to 1 mm diameter tip, and comes in three different lengths: 20, 30 or 40 cm. It is used together with a 10 mm diameter outer protecting nylon sheath [10] (Fig. 1 and Fig. 2). The medium sized catheter fits most small and medium sized bitches. The equipment can be obtained from the Norwegian Fur Breeders‘ Association, P.O. Box 136, Økern, N-0509 Oslo 5, Norway.

Figure 2. A close-up of the two sizes of tips of the Scandinavian AI catheter for dogs.
Intra-uterine AI with the Scandinavian catheter is performed with the bitch standing on the floor or on a table. Sedation is very rarely needed; on the contrary, most bitches in estrus freely accept this type of handling. In case a light sedation should be required for instance in a very large, obese or nervous bitch, 1 – 3 mg/kg xylazine IM or IV can be used. The inner steel catheter, with the tip within and protected by the nylon sheath, is introduced into the vagina. The cranial end of the nylon sheath is palpated in front of the pelvic brim as previously described. If the tip of the catheter sheath has been lowered closer to the abdominal wall the cervix usually is found a few cm in front of and above it. The steel catheter then is introduced through the sheath until its tip reaches the ventral fornix. The cervix is fixed between the thumb and the index finger and, by applying a slightly downward traction at the corpus uteri, it is tilted so that the angle of the cervical canal becomes more horizontal (Fig. 3).
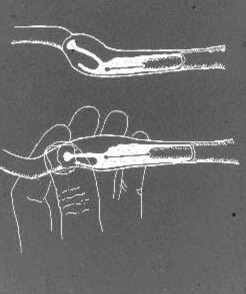
Figure 3. A schematic drawing of the canine paracervical region and the cervical canal, with the Scandinavian catheter in position in the cranial vagina. By manipulation at the cervix and corpus uteri the angle of the cervix is changed to get the cervical canal in better alignment with the tip of the catheter to facilitate the catheterization.
The tip of the catheter is then carefully withdrawn while pushing it repeatedly against the surface of the cervix in search of the opening of the cervical canal. The sensation when this opening is found can in most cases be described as the sensation of touching cartilage, i.e. ”crispy”. Once the opening has been found, fix the catheter and start working the cervix against the catheter. The cervical canal is 5 to 10 mm long, and not always completely straight. Thus, a slight pressure may have to be applied, while rotating the catheter to ease it through. In most bitches, the tip of the catheter easily can be felt in front of the cervix in the corpus uteri. In some bitches, however, the sensation is not as distinct. The syringe containing semen is connected to the catheter and the semen slowly infused into the uterus. Sometimes there is a resistance to infusion depending on whether the opening of the catheter is pressing against the endometrial mucosa. A light downward traction of the corpus uteri or the cervix usually alleviates the situation and allows semen infusion. To check that the catheter really is in the uterus of the bitch 1 – 2 ml of physiological saline can be infused. If the catheter is in the right position in the uterine body the fluid can easily be infused. If, on the other hand, the catheter is in the paracervical region, there will be an almost immediate backflow of saline between the catheter and the nylon sheath. The catheter is removed and the hindquarters of the bitch are elevated and the bitch kept in this position for 5 – 10 minutes after the AI to minimize backflow of semen and to facilitate uterine transport of spermatozoa toward the oviducts [7,11]. The bitch should also be feathered around the perineal region as this is believed to stimulate uterine contractions. To learn this technique requires some practice, but once learned it is a quick method, usually being accomplished within minutes. It is recommended that, initially, organ specimens be obtained for training purposes and anatomical study. It is also an advantage if especially the first attempts are made in bitches that have given birth to one or more litters, as they are usually much easier to catheterize. Perforations may occur if the catheter is introduced blindly or with force. Provided that the catheterization is performed under careful palpatory control, however, the technique is completely safe for the bitch. Some bitches are more difficult to catheterize, particularly those belonging to some of the giant breeds, as well as obese or nervous animals. Using the Scandinavian catheter only between 2 and 3.5% of attempts at intra-uterine catheterization were unsuccessful [3,6]. Resulting whelping rates using frozen-thawed semen has been reported to be 84.5% [4], and 71% [6] when performed by skilled inseminators, an average of 65% with fresh and chilled semen and 52% with frozen-thawed semen, in a larger field study involving also less-experienced inseminators [5] (Fig. 1). This technique can also be used for intra-uterine infusion of contrast medium for hysterographic examination of the bitch [12].
Intra-Uterine Insemination using Endoscopic Visualization of the Cervix
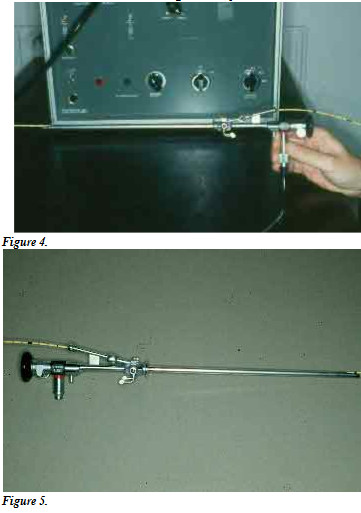
Transcervical intra-uterine insemination can also be accomplished with the aid of a rigid fiberoptic endoscope and a urinary or angiographic catheter, on the standing bitch, and without sedation (Fig. 4 and Fig. 5).
Intra-Uterine Insemination using Laparoscopy
Abdominal laparoscopy should offer a somewhat more acceptable alternative to full surgery for AI in the dog. The technique has been described by Wildt [15] and Silva et al. [16]. A 60 to 73% pregnancy rate has been reported by AI using laparoscopy [16,17,18], but the number of bitches in those studies were few.
Intra-Uterine Insemination using Surgery
Surgery to perform intra-uterine insemination has been reported [19,20,21]. Various surgical procedures have been used, with the bitch under general anesthesia and in dorsal recumbency. The ventral abdomen is clipped, and after routine surgical preparation a 4 -6 cm incision is made midway between the pubis and the umbilicus, through the linea alba. The uterus is elevated through the incision, and the needle of the syringe containing the semen is inserted into the lumen of the uterine body at a 45o angle with the bevel of the needle up. The semen is slowly injected into the uterus. It should flow easily with obvious distention of the uterine horns, or else the needle should be repositioned. A saline moistened gauze is held over the injection site after the needle is withdrawn. After 1 min the gauze is removed, the uterus replaced into the abdomen and the wound closed using routine methodology. To avoid backflow of semen the bitch should be positioned with its rear elevated as she recovers from anesthesia [20]. Around 60% pregnancy rate has been reported after surgical AI in the dog [19], but like with laparoscopic AI results are based on limited experimental studies and no field data are available for evaluation using either method. Whether it is ethically acceptable to resort to surgery to achieve pregnancies is debatable. The method, although advocated by some, is considered by many to be unethical and unacceptably stressful for the bitch. The risks for infection, etc. associated with surgery in general and the limited number of surgical AI’s that can be performed in a given bitch are two obvious disadvantages. The method is also costly and time-consuming.
References
1. Seager SWJ, Platz CC, Fletcher WS. Conception rates and related data using frozen dog semen. J Reprod Fertil 1975;45:189-192.
2. Linde-Forsberg C, Forsberg M. Fertility in dogs in relation to semen quality and the time and site of insemination. J Reprod Fertil 1989;39(Suppl): 299-310. – PubMed –
3. Linde-Forsberg C, Forsberg M. Results of 527 controlled artificial inseminations in dogs. J Reprod Fertil 1993;47(Suppl): 313-323. -PubMed –
4. Linde-Forsberg C, Ström Holst B, Govette G. Comparison of fertility data from vaginal vs intrauterine insemination of frozen-thawed dog semen: A retrospective study. Theriogenology 1999;52: 11-23. -PubMed –
5. Linde-Forsberg C. Fertility data from 2041 controlled artificial inseminations in dogs. In: Proceeding of the 4th Int Symp Canine Feline Reprod, Oslo, 2000, 120 p.(abstr.)
6. Thomassen R, Farstad W, Krogenaes, A, Fougner, J.A. and Andersen Berg K. Artificial insemination with frozen semen in the dog. A retrospective study. J Reprod Fertil 2001, in press.
7. Tsutsui T, Kawakami E, Murao I, et al. Transport of spermatozoa in the reproductive tract of the bitch: Observations through uterine fistulas. Jpn J Vet Sci 1989;51: 560-565. -PubMed –
8. Linde-Forsberg C. Artificial insemination with fresh, chilled extended, and frozen-thawed semen in the dog. Seminars in Vet Med Surg (Small Animal) 1995;10: 48-58.
9. Tsutsui T, Tezuka T, Shimizu T, Murao I, Kawakami E, Ogasa A. Artificial insemination with fresh semen in Beagle bitches. Jpn J Vet Sci 1988;50: 193-198.
10. Andersen K. Insemination with frozen dog semen based on a new insemination technique. Zuchthyg 1975;10: 1-4.
11. Linde C. Transport of radiopaque fluid into the uterus after vaginal deposition in the oestrous bitch. Acta vet scand 1978;19:463-465.
12. Funkquist B, Lagerstedt A-S, Linde C, Obel N. Hysterography in the bitch. Vet Radiol 1985;26:12-18.
13. Wilson M. Non-surgical intrauterine artificial insemination in bitches using frozen semen. J Reprod Fertil 1993; 47 (Suppl): 307-311. -PubMed –
14. Battista M, Parks J, Concannon PW. Canine sperm post-thaw survival following freezing in straws or pellets using PIPES, lactose, tris or TEST extenders. In: Proceedings of the 11th Int Congr Anim Reprod and AI, Dublin 1988;3: 229-231.
15. Wildt DE. Laparoscopy. In: Burke TJ, ed. Small Animal Reproduction and Infertility. Philadelphia: Lea & Febiger, 1986;121-140.
16. Silva LDM, Onclin K, Snaps F, Verstegen JP. Laparoscopic intrauterine insemination in the bitch. Theriogenology 1995;43: 615-623.
17. Silva LDM, Verstegen JP. Comparisons between three different extenders for canine intrauterine insemination with frozen-thawed spermatozoa. Theriogenology 1995;44: 571-579.
18. Silva LDM, Onclin K, Lejeune B, Verstegen JP. Comparisons of intravaginal and intrauterine insemination of bitches with fresh or frozen semen. Vet Rec 1996;138: 154-157. – PubMed –
19. Smith FO, Graham EF: Cryopreservation of canine semen: Technique and performance. In: Proceedings of the Xth Int Congr Anim Reprod and AI, Champaign-Urbana 1984;2: 216.
20. Hutchison RV. Vaginal & surgical intra-uterine deposition of semen. In: Proceedings of the Canine Theriogenology Short Course 1993; 33-37.
21. Hutchison RV. Maximizing conception rates using fresh cooled or frozen canine semen. In: Proceedings of the Canine Male Reprod Symp 1997; 61-70.
All rights reserved. This document is available on-line at www.ivis.org. Document No. A1207.0201.

